advancing the mitigation of Climate Change and Global Warming through Geoengineering education and research
Carbon Capture and Storage
Carbon Capture and Storage or CCS is a geoengineering or climate engineering approach that reduces carbon dioxide emissions by capturing carbon dioxide and permanently storing it deep underground. Carbon Capture and Storage approaches focus on reducing the carbon dioxide that is emitted by fossil fuel-based energy producers such as coal power plants (Please see PBS NewsHour’s YouTube Video Could carbon capturing make “clean coal” a reality?).



How does Carbon Capture and Storage work?
The geoengineering process of carbon capture and storage involves isolating CO2 from fossil fuel power stations in pre or post-combustion processes, compressing the CO2 until it forms a liquid, transporting the liquid CO2, often through pipelines, and then pumping it deep underground into porous rock layers that can safely hold it for long periods of time (Please see the Minerals Council of Australia’s YouTube video below “How does Carbon Capture and Storage work?”).
Newgencoal.com.au’s YouTube video “How does Carbon Capture and Storage work?” Video courtesy of the Minerals Council of Australia.



How is carbon dioxide captured at coal or other fossil fuel power stations?
Carbon or CO2 Capture is accomplished through pre-combustion and post-combustion processes.
Pre-combustion CO2 capture is carried out through a process called gasification (Please see the National Energy Technology Laboratory’s YouTube Video below “Gasification Animation”). In the gasification process, fossil fuels and other hydrocarbon-based fuels are heated with a little oxygen and without combustion (combustion is the burning of a fuel in the presence of oxygen) to produce a gas mixture called Syngas which primarily consists of carbon monoxide (CO) and molecular hydrogen (H2). The Syngas is then transported to a shift reactor where, in the presence of steam (known as steam reforming), the Syngas is converted into a gas mixture that consists of H2 (a high-quality fuel) and CO2 through what is called the water-gas-shift reaction. The H2 and CO2 gas mixture is then transported to an absorber tower where an amine-based (-NH2) solvent reacts and combines with the CO2. This process is known as amine gas treating or amine scrubbing. The amine solvent-CO2 solution then enters a stripper tower where the solution is heated to separate the CO2 gas from the amine solvent. After the mixture is heated, the CO2 gas floats out of the top of the stripper tower and the amine solvent flows out of the bottom of the tower where it is collected and reused (Please see minute 3:31 of PBS NewsHour’s YouTube Video above for an explanation of the amine scrubbing process).
National Energy Technology Laboratory’s YouTube Video “Gasification Animation, A short explanation of coal gasification”.
Post-combustion CO2 capture occurs after the fuel has been burned with air. This process can be retrofitted to existing power plants. After combustion, the flue gas which mostly consists of water, nitrogen and CO2 enters an absorber tower where the CO2 is chemically combined with an amine-based solvent in the process of amine gas treating or amine scrubbing. Similar to the pre-combustion process, the CO2 is then separated from the amine solvent-CO2 solution in a stripper tower.
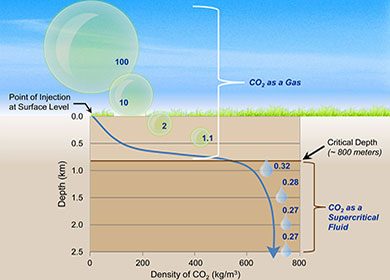

How is captured carbon dioxide stored or sequestered?
Carbon dioxide storage involves the process of isolating captured CO2 and preventing the gas from re-entering the atmosphere. Storage approaches include incorporating the CO2 in products such as concrete and/or storing it underground. Storage of captured CO2 underground involves compressing the gaseous CO2 and converting into a liquid. The liquid CO2 is then transported (typically pumped through pipelines) to well fields. The liquid CO2 is then pump down deep drilled wells (or existing wells) below non-porous rock layers. Once below stable non-porous rock layers or geological formations, the liquid CO2 is pumped into porous rock layers where it is theoretically stored for periods on the order of thousands of years (Please see the National Energy Technology Laboratory’s YouTube Video below “The Energy Department and NETL’s Advances in Carbon Capture and Storage”).
National Energy Technology Laboratory’s YouTube Video The Energy Department and NETL’s Advances in Carbon Capture and Storage.


